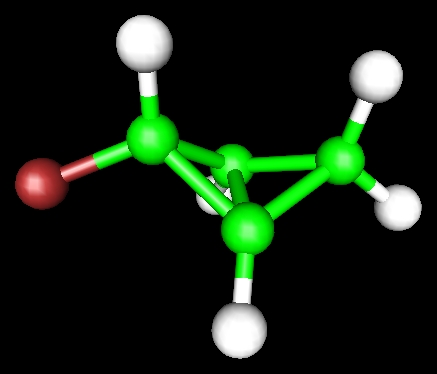
Electronic structure can be used to derive lots of information concerning chemistry.
Imagine you wanted to make 2-Fluoro-Bicyclobutane. Using methods based on the theory of electronic structure, you can predict:
Its structure:

The central Carbon-Carbon bond has a length of 1.52 Angstrom - not too stretched
Its stability. It is roughly 23 kcal/mol more stable than two possible (but not very plausible...) starting products, acetylene and fluoroethylene.
Its spectroscopy. The C-F bond has a predicted frequency of 1184 cm-1, and the normal mode looks like this:
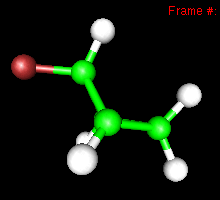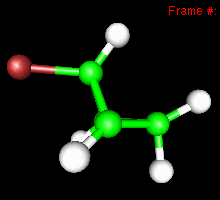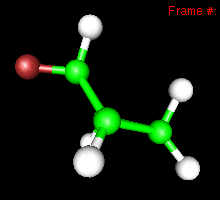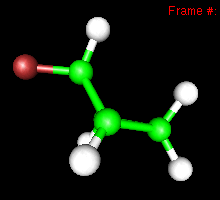
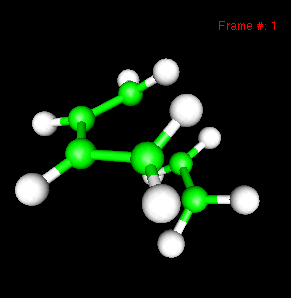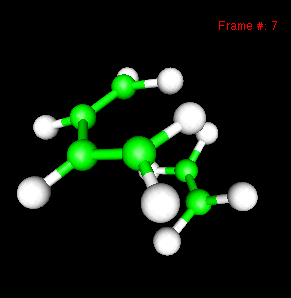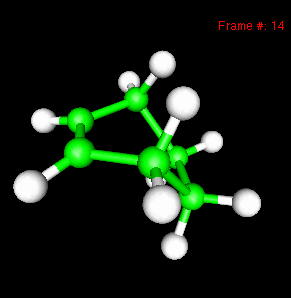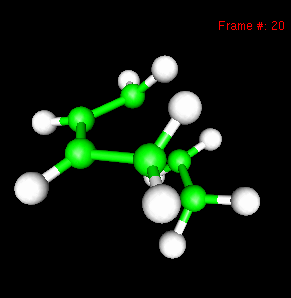
You can also study reactivity, for example by locating transition states. Here, you can see the reaction coordinate at the transition state of the Diels-Alder reaction:
Or you can predict reactivity by examining the orbitals of the reagents. Here, for example, is what the lowest unoccupied orbital of the Tungsten complex W(CO)5[=CH(OCH3)] looks like:
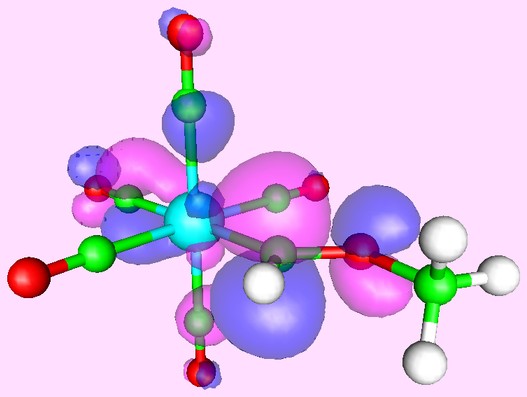
Clearly, the best site for attack of a nucleophile is on the carbene carbon atom - which is exactly what is found experimentally.
Back to the menu.
Forward to the first lecture.